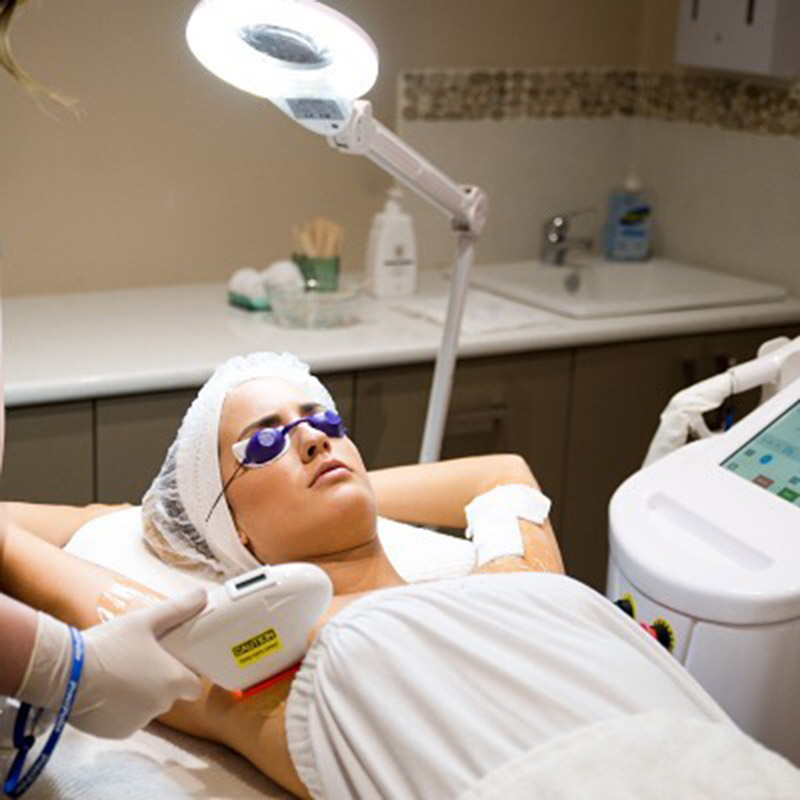
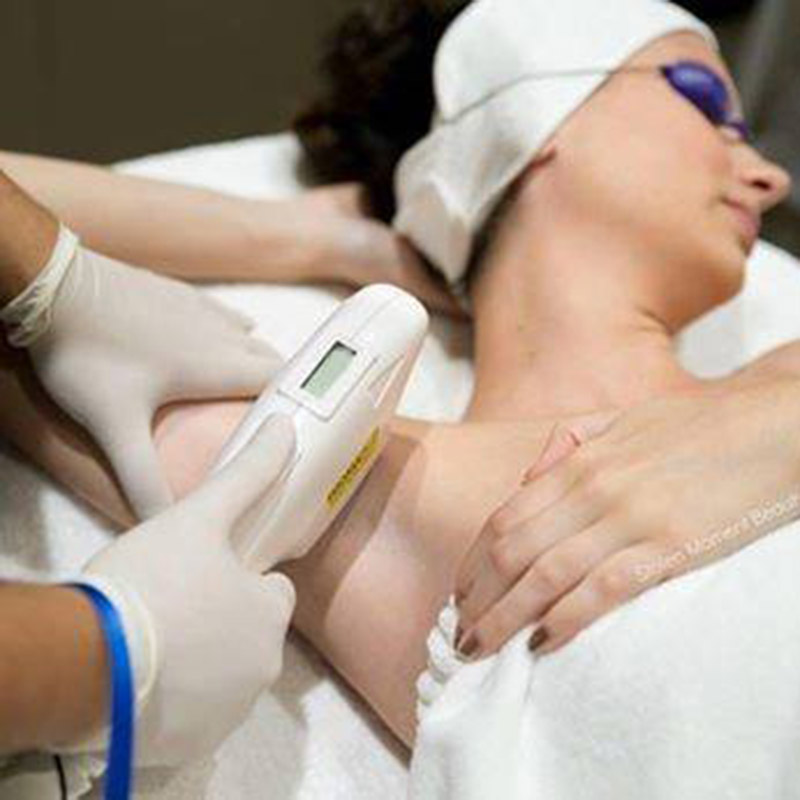
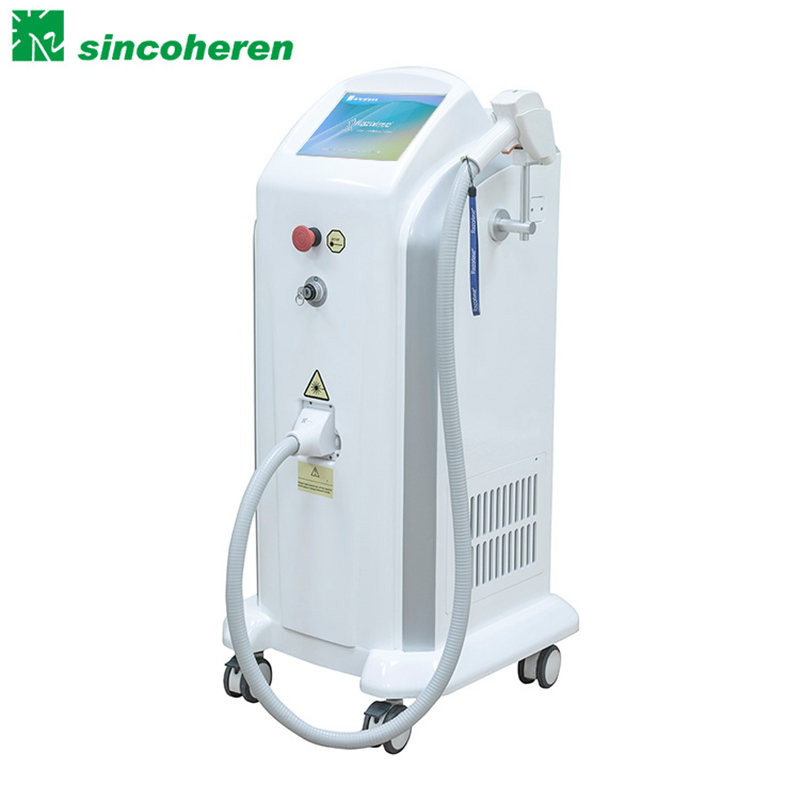
ʻO China wholesale Fda Approved Machine - FDA a me TUV Medical CE i ʻae ʻia SHR IPL Device - Sincoheren
ʻO China wholesale Fda Approved Machine - FDA a me TUV Medical CE i ʻae ʻia ʻo SHR IPL Device - Sincoheren Detail:
Nā noi
1, Wehe lauoho mau
2, Hoʻoulu hou i ka ʻili
3, wehe ʻia ka pigmentation
4, ka wehe ʻana i ka ʻāʻī
5, Ka wehe ʻana i ke koko, Rosacea, wehe ʻia nā vein ʻulaʻula
Nā pono
1.Smart a kū hoʻokahi i hana lima lima.
2. Aia ma lalo o ka ikehu hoʻomalu ʻia, lawe mai ʻo USA i ka pauma hoʻoluʻu
3. ʻOkoʻa refrigeration pono, hōʻoia eha noa lapaʻau
4. ʻO ke ʻano hana akamai a maʻalahi.
5, Ka nui wahi nui.
6, Hoʻolālā UI flattening a me ka hoʻonohonoho maʻalahi o nā ʻāpana
7, ke ola lima lima ma luna o 300000-500000 kiʻi
Nā kikoʻī
Kumu kukui: kukui Xenon
Spectrum: HR 690-1200nm SR 560- 1200nm
Ka lōʻihi o ka Pulse: 20- 149.7ms
Kaʻina Pulse:2 a i ʻole 3 Pulse
Hana hou: 1-10Hz
ʻO ka ikaika o ka ikehu: 20- 50J/cm2
Ka nui wahi:16×57 mm no HR 8×34 mm no SR
Pūnaehana hoʻoluʻu: Semiconductor hoʻoluʻu, hoʻoluʻu wai i kūkulu ʻia a me ka hoʻoluʻu ea E hoʻopili i ka ʻenehana hoʻoluʻu ʻili.
Hoʻoluʻu ʻili
Pono uila:230VAC 50Hz 1200W
Ana (WxDxH):46cm*75cm : * 40cm
Kaumaha ʻupena:36kg
Palapala hōʻoia: FDA a me CE
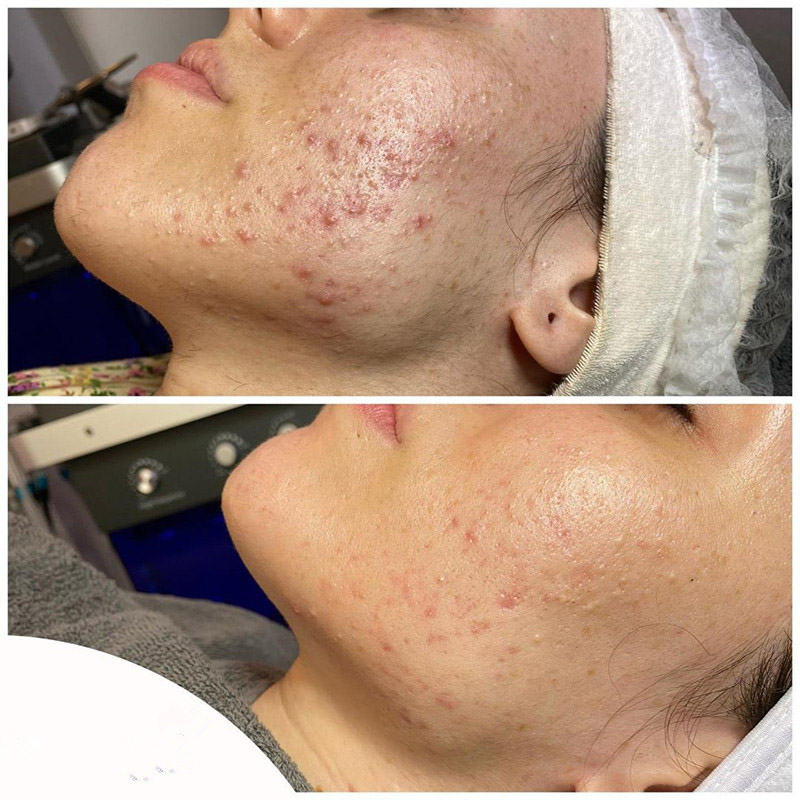
Ka hopena lapaʻau
Nā kiʻi kikoʻī huahana:
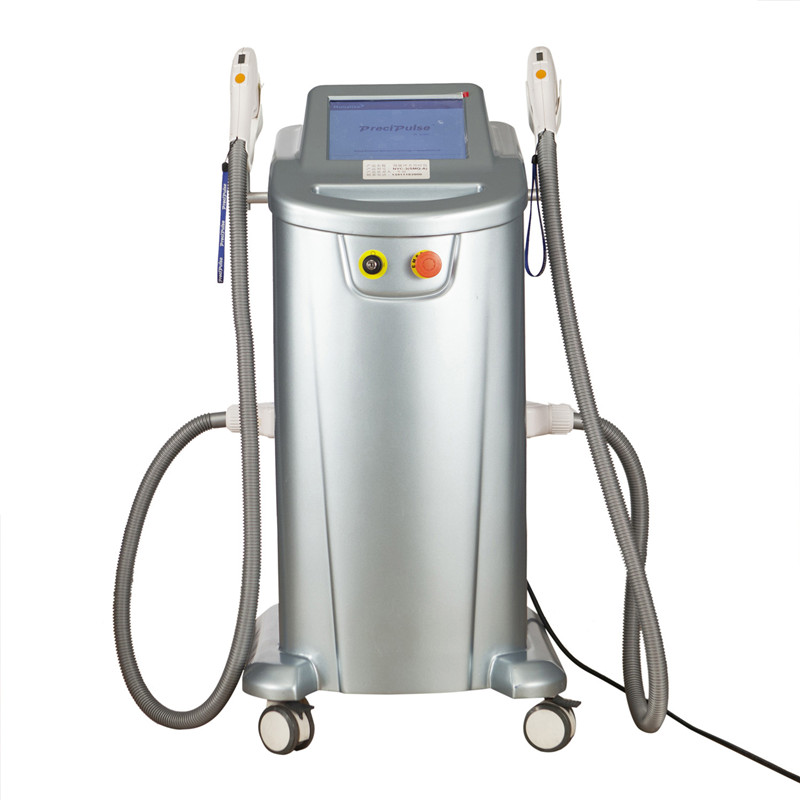
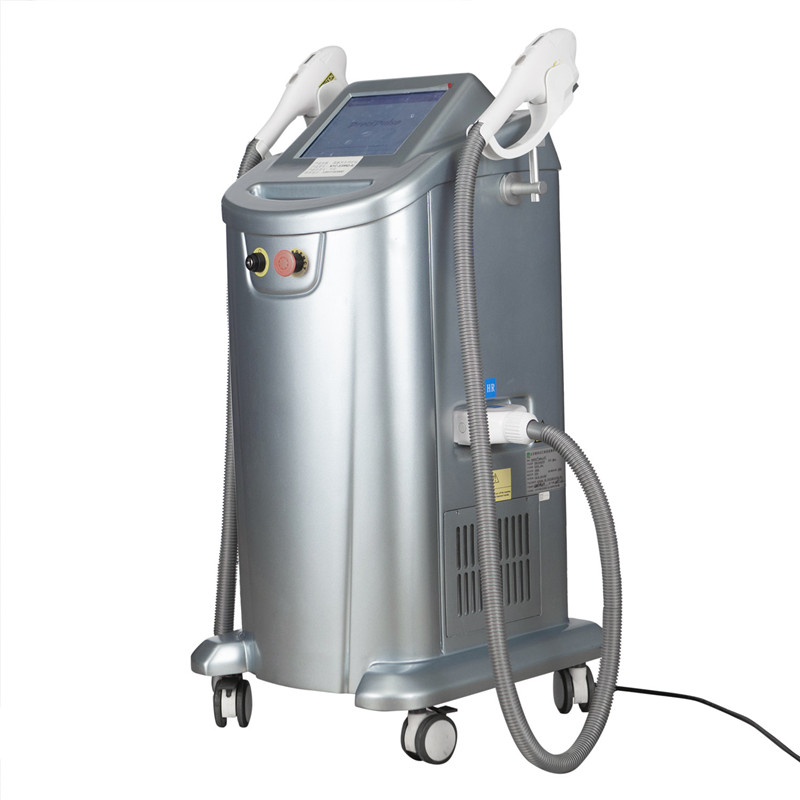
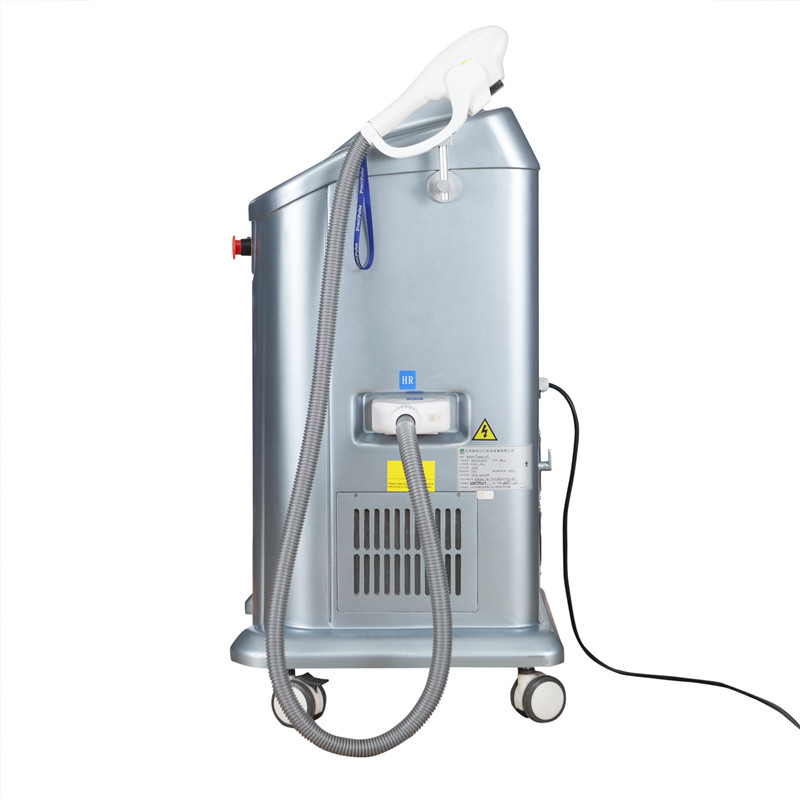

Alakaʻi Huahana Pili:
Loaʻa iā mākou kā mākou mau limahana kūʻai huahana ponoʻī, nā hui kaila, hui ʻenehana, nā limahana QC a me nā limahana pūʻolo. Loaʻa iā mākou nā kaʻina hana hoʻokele waiwai kiʻekiʻe no kēlā me kēia ala. Eia kekahi, ua ʻike kā mākou mau limahana āpau i ka paʻi kumuhana no Kina wholesale Fda Approved Machine - FDA a me TUV Medical CE i ʻae ʻia SHR IPL Device - Sincoheren , E hoʻolako ka huahana i nā wahi āpau o ka honua, e like me: Mumbai , Montpellier , Egypt , Mai mau, mākou adhering i ka hamama a me ka pono, kaʻana like e loaʻa, i ka imi ana i ka maikaʻi, a me ka hana ana i ka waiwai waiwai, pili i ka pono a me ka efficient, kalepa-oriented, ala maikaʻi loa , maikaʻi valve pāʻoihana philosophy. Me ko mākou honua holoʻokoʻa he mau lālā a me nā hoa hana e hoʻomohala i nā wahi ʻoihana hou, nā kumu waiwai maʻamau. Aloha nui mākou a hui pū mākou i nā kumuwaiwai honua, e wehe ana i kahi ʻoihana hou me ka mokuna.
 Na Nicci Hackner mai Nigeria - 2017.06.19 13:51
Na Nicci Hackner mai Nigeria - 2017.06.19 13:51 He mau hoa ʻoihana maikaʻi loa ia, e kakali ana i ka launa pū ʻana aʻe!
 Na olivier musset mai ka ʻIseraʻela - 2018.09.21 11:44
Na olivier musset mai ka ʻIseraʻela - 2018.09.21 11:44